Chemical Bonding and Shape of Molecules
Questions and Answers
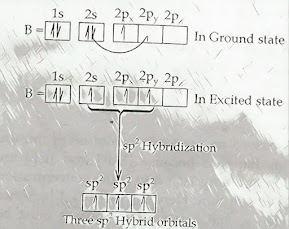
1. How would you confirm that B in BF3 gets sp2 Hybridization?
> The central atom Boron has one half filled orbital in ground state (1s12s22px12py02pz0) but it has three half filled orbital in excited state since one electron is promoted from 2s to 2p orbital during chemical combination.
Hence, one 2s and two 2p orbital undergo sp2 hybridization to
form three Sp2 hybrid orbital of equivalent energy and identical
shape & size which takes trigonal planer geometry with bond angle 1200.

2. C in C2H2 gets
sp hybridization, why?
>Each carbon has two half filled orbitals in ground state (1s22s22p×12py12pz0).It has four unpaired electrons in excited state one electron is promoted from 2s to empty 2p orbital during chemical reaction (1s22s12px12py12pz1). One 2s and one 2p orbitals undergoes sp hybridization to form two sp hybrid orbitals of equivalent energy and identical shape which has linear geometry and bond angle 1800.
One half filled sp hybrid orbital of carbon atom overlaps with another half filled sp hybrid orbital to form sigma bond. The remaining half filled Sp hybrid orbital of each carbon atoms overlaps with half filled orbitals hydrogen to form sigma bond. The unhybridized orbital of carbon atoms pi bond.

>The necessary condition for hybridization:
1. The orbitals taking part in hybridization must have only small difference of enthalpies.
2. The orbital undergoes hybridization generally belong to the valence of the atom. The mode of hybridization of C in C2H2 is sp-hybridization.
4. Predict the mode of hybridization of B in BF3. Mention any two features of this hybridization.
->The central atom B in BF3 has Sp2 hybridization. Two important features of this hybridization are:
1. Bond angle of Sp2 hybrid orbital is 1200.
2. Sp2 hybrid orbital having 33% s-character and 66% p-character.
5. Predict the model of hybridization. i)Carbon in ethane ii)oxygen in water
> i) The hybridization of carbon in ethyne is p-hybridization.
ii) The hybridization of oxygen in water is sp3-hybridization.
6. Write two important features of hybrid orbitals.
> The two important features of hyhbrid orbitals are;
i) They have same shape and same energy.
ii) They are equivalent and symmetrical.
7. Which kind of hybridization result into tetrahedral geometry? Give an example of such hybridization.
-> sp3 hybridization results into tetrahedral geometry.
Example: methane (CH4)
> sp hybridization results into linear geometry.
Example: - Ethyne (C2H2) has sp hybridization.
> Sp2 hybridization results into trigonal planer geometry. Example: ethene (CH2=CH2).
> According to VSEPR model, molecules takes a shape that minimizes the repulsion between bonding pair i.e. bond pair and non-bonding i.e. lone pair of electron.
Lone pair-bond pair i.e. bond pair repulsion>bond pair-bond pair repulsion
In NH3 molecule, three bonding pair of electron and one lone pair of electron are present. Due to the presence of lone pair of electron the regular tetrahedral structure of NH3 is distorted to give triangular pyramidal shape and having bond angle 106045'. This gives the maximum stability between the bond pair and lone pair of electrons.

> The central atom N has one lone pair and three bonding electrons. Three bonding electrons are shared with three hydrogen atom to form three bond pair electrons. According to VSEPR theory, lone pair-bond pair electros. According to VSEPR theory, lone pair-bond pair repulsion is greater than bond pair-bond pair repulsion. Due to the unequal repulsion, tetrahedral geometry is distorted

12. Predict the mode of hybridization in the central atom of the molecules having
(i) trigonal planner (ii) tetrahedral structure with an example of each.
>i) Trigonal planner: The mode of hybridization in certain atom is sp2. Example: BF3
ii) Tetrahedral: The mode of hybridization in centre atom is Sp3.
Example:- CH4
13. Define hybridization and write any two features of tetrahedral hybridization.
> The process of mixing of dissimilar atomic orbital of same atom giving rise to equal number of new set of orbital having same energy is known as hybridization.
Features of tetrahedral hybridization.
Two important features of tetrahedral hybridization are:
a. Carbon atoms are sp³ hybridization.
b. The bond angles are 109.5°.
14. what are the features of. Tetrahedral hybridization? Write an exam of it.
a. Carbon atoms are sp³ -hybridiszation.
b. The bond as angles are 109.5°.
Example: Methane (CH4), the central carbon atom is sp³ hybridization.
15. state the mode of hybridization in B of BF3 and C of C2H6.
> The mode of hybridization in B of BF3 is sp² hybridization and mode of hybridization in C of C2H6 is sp³.
16.Which kind of hybridization results into tetrahedral geometry? Mention any one character of such hybridization.
> Sp³ hybridization have tetrahedral geometry. sp³ hybridization is formed by mixing of one s-orbital and three p-orbital. The characteristics of sp³ hybrid orbital: it has bond angle 109.5° and tetrahedral shape.
17.Which kind of hybridization results into tetrahedral geometry? Mention any one character of such hybridization.
> The mode of hybridization in carbon of acetylene is sp.
Two important features of sp-hybridization are:
i. The bond angle are 180°.
ii. The space sp-hybridization is linear, sp hybridization orbital possess 50% s-character and 50% s-character and 50% p-character.
18. Predict the geometry of molecules having:
a. sp³ hybridization
b. so hybridization with am example of each.
> a. sp³ hybridization:- The molecules having sp³ hybridization generally possess tetrahedral shape. Example:- CH4 molecule.
b. so hybridization:- The molecules having sp hybridization generally possess linear shape.
Example:- CH2H4 molecule.
19.write any two features of sp³ hybrid orbital with an example.
> Two features of sp³ hybrid orbital are:
i. The bond angle of sp³ hybrid orbital is 109.5° and tetrahedral shape.
II. sp³ hybridization orbital possesses 25% s-character and 75% p-character.
Methane molecules has sp³ hybrid orbital.
20.what is meant by hybrid orbital? Write an example of it.
> Hybrid orbital:- The process of mixing of dissimilar atomic orbitals of same atom giving hybridization and new orbital is called hybrid orbital.
Example:- Mixing of one s-orbital with one p-orbital to give two sp-hybrid orbital.

21.Why do NH3 and BF3 have dissimilar geometries?
>In NH3 molecule, the centaral atom N has one lone pair and three bonding electrons. These three bonding electrons is shared by three hydrogen atom to form three bonding pair of electrons. But in the case of BF3 molecule, central atom do not have lone pair electron and only form three covalent with three F atom. Due to the lone pair and bond pair repulsion, geometry of NH3 and BF3 are different.
22.State the mode of hybridization of C2H5 and mention any two features of this hybridization.
>Ethane molecule i.e. C2H6
has sp3 hybridization. Two features of sp3-hybridization are:
i. The bond angle of sp3 hybrid orbital is 109.5 degree and tetrahedral shape.
ii. sp3 hybridized orbital posses 25% s-character and 75% p-character.
Following are two important characters :
i. Carbon atoms are sp3-hybridization.
ii. The bond angle are 109.5 degree.
24. Mention one example of each:
i. In
methane (CH4), the central carbon atom is sp3-hybridizaton I.e.
tetrahedral shape.
ii. In boron trifluoride (BF3), the central boron atom has trigonal hybridization.
25.What is the mode of
hybridization of the central atom whose molecular geometry is tetrahedral? And
give an example of its.
>If the molecule has
molecular geometry of tetrahedral, the central atom of the molecule is sp³ hybridization.
Examples : In CH4 molecule, the central carbon atoms are
insp³ hybridization and having bond angle 109.5 degree.

26. predict the mode
of hybridization in
i. C of C2H4 ii. B of BF3
> I. the mode of hybridization is C of CH2H4 is also SP2 hybridization.
Ii. The mode of hybridization in B of BF3 is also SP2 hybridization.
27. Why is H-O-H bond angle
in water molecules comparatively higher than H-S-H bond angle in H2S
molecule?
> In both molecules i.e. H2O and H2S, the central atom O- and S- are SP3 hybridized orbital and both two having two bond pair and two lone pair of electros . O- atom has similar size and higher electronegativity than S- atom, due to this reason bond pair in H2O are closer to the oxygen atom and repulsion between them are large. In the case of H2S , the bond pair are away from the central atom. The bond angle in water molecule is comparatively higher than H2S.

28. Define hybridization. Draw the
orbital picture of a hydrocarbon showing tetrahedral structure.
> The process of mixing of
dissimilar atomic orbitals of same atom giving, rise to equal number of a new
set of orbitals having same energy is known as hybridization. Methane shows
tetrahedral structure and orbital picture of methane is

> According to VSEPR model, a molecule takes a shape that minimizes the repulsion between bonding pair i.e. bond pair and non-bond pair of electron.[ Lone pair-bond repulsion> bond pair-bond pair repulsion] In NH3 molecule, three bonding pair of electrons and one lone pair of electrons are present. Due to the presence of lone pair of electrons the regular tetrahedral structure of NH3 is distorted to give triangular pyramidal shape and having bond angle 106degree 45min. This gives the maximum stability between the bond pair and lone pair of electrons.

30. The bond angle at the central atom in NF3 is 103degree, whereas in BF3 is 120degre, what factor accounts for the difference in bond angles?
> Due to the presence of lone pair
of electrons in central atom N in NF3, the lone pair-bond pair
repulsion is greater than bond pair repulsion. The shape of NF3 is
pyramidal trigonal geometry. Due to the absence of lone pair of electron B in BF3,
there is only bond pair-bond pair repulsion and having triangular geometry with
bond angle 120 degree. Therefore, NF3 has pyramidal trigonal and BF3
has triangular geometry.

> All the four carbon atoms in methane are sp³ hybridization which are directed towards the four corners of regulars tetrahedral having bond angle of 109.5 degree. Each bond is formed by the head overlapping at sp³ shybrid orbital of carbon and S-orbital of hydrogen. Therefore, all the four C-H bond methane are identical.
32. Draw the molecular orbital picture of ethane.
> Molecular orbital picture of ethene is given below:

> Orbital picture of ethyne indicating sigma and pi-bonds is given below:

34.Draw the shape of sp and sp2 hybrid orbitals.
> when one S-orbital and one p-orbital are mixed together then two sp-hybrid orbital is formed. The shape of sp-hybrid orbital is linear .i.e. bond angle 180 degree.

> when one s-orbital and two p-orbital are mixed together then three sp2- hybrid orbital is formed. The shape of sp2-hybrid orbital is trigonal i.e. bond angle 120 degree

35. Identify the hybridization of the indicator atom in each of the following molecules.
A Be in BeF2 b. B in BF3 c. N in NH
> Hybridization of Be in BeF2 is sp-hybridization.
Hybridization of B in Bf3 is sp² hybridization.
Hybridization of N in NH3 is sp³ hybridization.
36. Predict the structure of methane based on hubridization.
> The electronic configuration of central atmo C in methane is
C6=1s²2s²2p²(ground state)
The P-orbital is empty. During bond formation, carbon atom absorbs energy and one 2s
electron gets promoted to 2p orbital.
C6=1s²2s¹2p³(excited state)
These four sp³ hybrid orbital overlap with 1s orbital of 4 hydrogen atoms to give a methane molecule. Thrse four bondsare directed towards the corners of a regular tetrahedral with bond angle of 109.5°.
figure:-

37. Explain the state of hybridization in
ethyne molecule.
> The central carbon atom in ethyne and one p- orbital of the exicted carbon atom hybridize to form two sp hybrid orbital. Two sp hybrid orbital are inclined to each other at right angle i.e bond angle 180°. In
ethyne, each carbon atom forms two sigma bond using sp hybrid orbital and two pi bonds are formed by using unused p orbitals of carbon atim. The orbital pictures of ethyne is

38. What do you understand
by sp² hybridization ? Using any example explain the molecular geometry
involved.
>
sp²-hybridization: When one s-orbital and two p-orbital mix together to form
three sp²-hybrid orbital, is called sp²-hybridization. The three hybrid
orbitals are oriented to the three conrers of a triangle having an angle of
120° to one another.

Ethene molecule has sp² hybridization. In ethene molecule the three sp2 hybrid orbitals form sigma bonds, while the unused p-orbital will form π bond. The orbital picture of ethene molecule is

39. Using VSEPR theory, explain the shapes of BeF2 and BF2.
> In BeF2 molecule, the central atom Be have two valence
electrons. These two elections are shared by two F atoms and two bond
pair of electrons are formed around the central atom Be. According to VSEPR
theory, these two bond pair of electron are arranged in such a way that there
must be maximum repulsion. These two bond pair is in 180° apart from each
other. The geometry of BeF2 is linear.
In BF3 molecule, the central atom B has three valence electrons. These three electrons are shared by three F atom to form three bond pair of electrons. They have equal force of repulsion among each other and are oriented in triangular geometry with bond angle 120°.

40. VSEPR model
> Valence shell
electron pair repulsion theory is also called VSEPR theory. This theory was
given by Sidgwick and Powell. This theory describes the influence of lone pair
of electrons to the bonding electrons and also predicts the shape of molecule.
This theory is based on the number of valence of electrons present in the
central atom of a molecule.
Statement: "A
molecules take a shape that minimizes the repulsion between the bonding and
non-bonding pair of electrons".
Following are the
postulates of VSEPR theory.
a. In any covalent
molecule, there is a central atom around which other atoms are arranged.
b. The shape of
molecule is determined by the number of electrons pair presents in the valence
shell of the central atom.


Badhiya
ReplyDelete