Chemical Bonding and Shape of Molecules
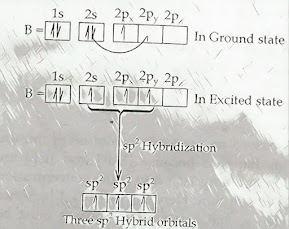
Questions and Answers 1. How would you confirm that B in BF 3 gets sp 2 Hybridization? > The central atom Boron has one half filled orbital in ground state (1 s 1 2 s 2 2px 1 2py 0 2pz 0 ) but it has three half filled orbital in excited state since one electron is promoted from 2s to 2p orbital during chemical combination. Hence, one 2s and two 2p orbital undergo sp 2 hybridization to form three Sp 2 hybrid orbital of equivalent energy and identical shape & size which takes trigonal planer geometry with bond angle 120 0 . Each half filled sp 2 hybrid orbital of boron overlaps with half filled 2p z orbital of three fluorine atom to form three covalent bonds. 2. C in C 2 H 2 gets sp hybridization, why? >Each carbon has two half filled orbitals i...